THE VALUE OF IVDs

What is an IVD?
‘In vitro’ is Latin for ‘in glass’. IVDs are tests performed on a sample taken from the body to diagnose a disease or condition. Samples can include blood, urine, or tissues. Classically, these tests were carried out in a test tube or on a laboratory dish, hence the name ‘in vitro’. While this term might seem old-fashioned in the post-COVID world, where most of us are accustomed to home testing with lateral flow devices, it is well understood within the life science and healthcare worlds.
IVDs vary from sophisticated technologies performed in clinical laboratories by highly-trained scientists to simple self-tests conducted by the public. IVDs can be performed at the point-of-care (POC), within clinical settings, or by individuals themselves at home, for example self-monitoring of blood glucose for management of diabetes.

The Statistic
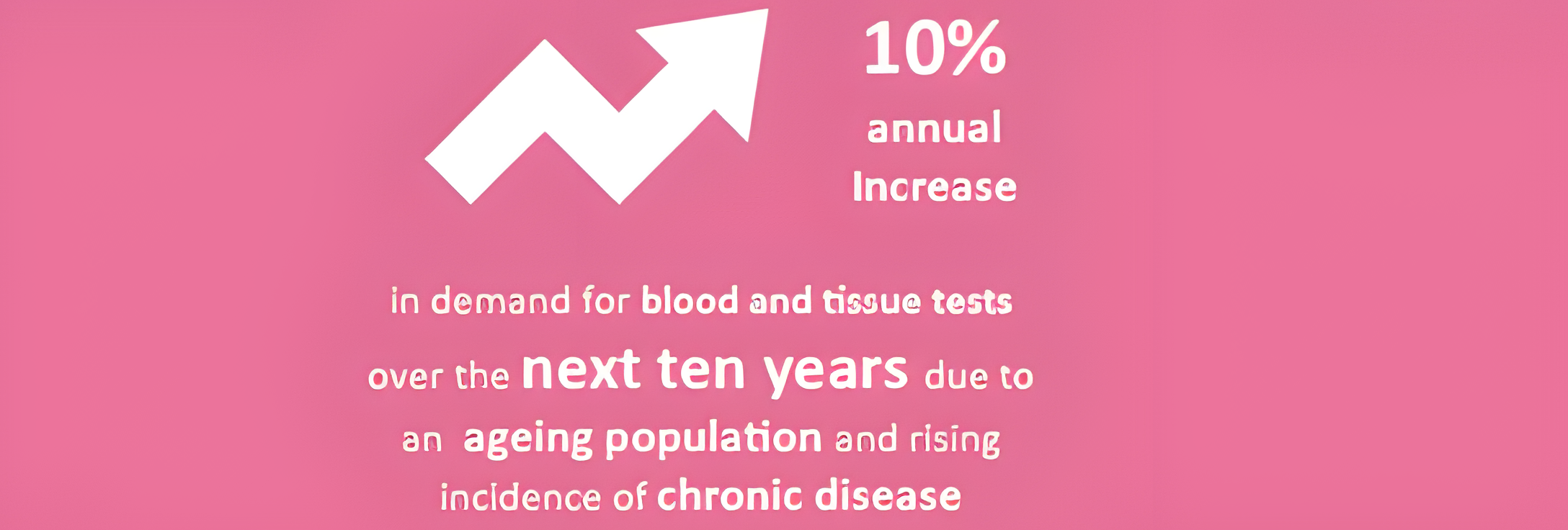
The life sciences sector almost always uses the statistic that approximately 70% of clinical decisions are influenced by the use of in vitro diagnostics. Comparatively, approximately less than 1% of the NHS budget is dedicated to the uptake of new and innovative IVD products. Why is this? Could it be that the true value of IVDs has not yet been realised?
The Statistic
In vitro diagnostics (IVDs) are an essential part of the NHS. They are used to both enable diagnosis and to rule out causes of ill health. They are also used to monitor, screen and assess people for any potential health problems they might have. Increasingly, they also allow people with chronic disease to manage their own conditions.