Insights and Analysis

BIVDA regularly produces insight and analysis on the key issues impacting the IVD industry. You can learn more about some of these issues below:

Brexit
As the UK negotiates a new relationship with the EU, it is important to ensure that the environment for IVDs and life sciences as a whole remains as attractive as possible.
BIVDA has produced a report, informed by our members, which aims to provide a clear view about the aspects of the current environment that should be protected, as well as the opportunities that might exist for positive change. BIVDA has also been involved in the work of the UK EU Life Sciences Steering Committee and has aligned its recommendations with the four key topics that have been outlined by the group: innovation, people, regulation and fiscal and trade.
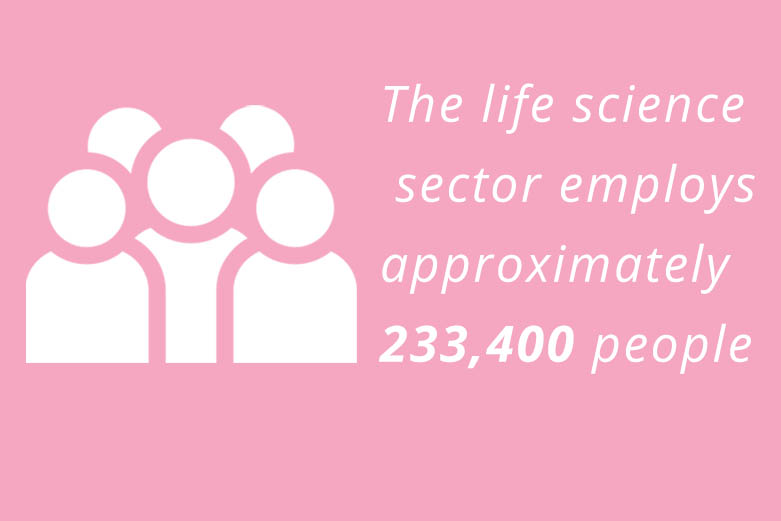
Life Sciences Sector Deal
The publication of the Government’s Life Sciences Sector Deal at the end of 2017 was a key moment for the IVD industry against a wider backdrop of change. Following its release, BIVDA issued the following comment:
“The life sciences industry is a jewel in the UK’s crown and I am delighted that the Industrial Strategy includes a number of strong recommendations to enable the in vitro diagnostics (IVD) sector to play its part in delivering benefits for patients, the NHS and UK economy.
Diagnosis led healthcare is the key to better outcomes and I hope the Government will implement the recommendations contained in the Strategy as soon as possible."
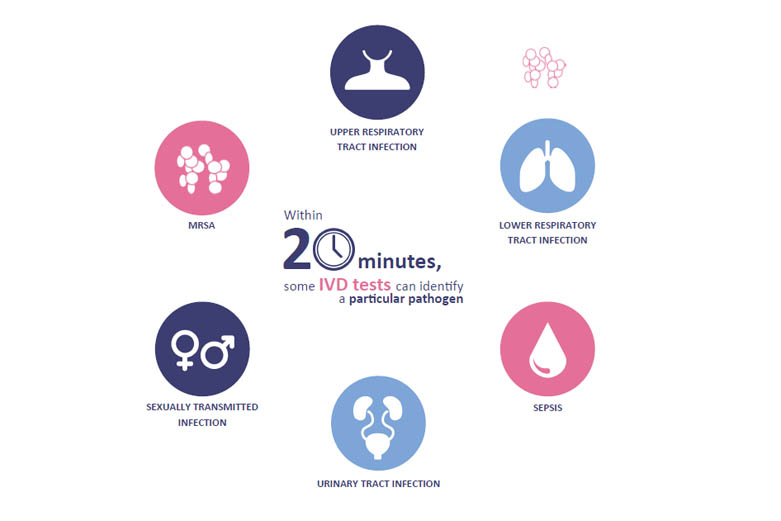
Antimicrobial Resistance
The importance of IVDs in tackling the antimicrobial resistance (AMR) challenge cannot be overstated.
While the widespread use of broad-spectrum antibiotics has helped to create multi-drug resistant strains of bacteria, greater adoption of IVDs would lead to a reduction in cases of unnecessary antibiotic prescribing by:
- Reducing time to appropriate treatment decision
- Allowing for a more targeted use of therapy against infection
Urgent action is required to halt AMR and we strongly urge healthcare professionals in the UK to make use of the quick, accurate and effective IVDs already available to them. Until IVDs are more widely adopted, we will not be able to combat AMR effectively. BIVDA members have produced a Declaration on the actions that need to be taken by Government and the industry to overcome the AMR challenge.